Abstract
Introduction
Pediatric inspired regimens can achieve good outcomes in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL) by delivering intense non-myelosuppressive chemotherapy and are considered standard. Experience with the implementation of these regimens outside of academic centers in high-income countries is limited. Furthermore, Mexican patients (with "hispanic ethnicity") have increased risk for relapse and treatment related complications.
Objectives
Primary objective: to determine 2-year overall survival (OS) and event-free survival (EFS). Secondary objectives: to determine the impacts of treatment abandonment and measurable residual disease (MRD) on outcomes, and to compare treatment costs with a widely used standard regimen in the United States.
Patients and Methods
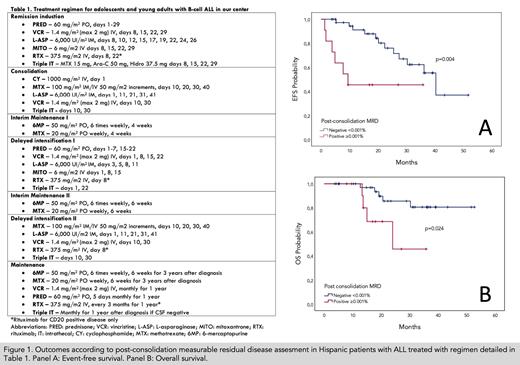
Consecutive patients 16-45 years diagnosed with Ph-negative B-cell acute lymphoblastic leukemia after 2016 were included. The salient features of our modified-BFM regimen include the use mitoxantrone, E. coli L-asparaginase, without systemic cytarabine or high dose methotrexate designed to be delivered entirely in an outpatient basis for maximum affordability (Table 1), given that we treat an uninsured population that must cover their own treatment costs out of pocket. Genetic risk assessment was limited to BCR/ABL. Thereafter, relapse risk assessment was based exclusively on "next generation" flow cytometry measurable residual disease (MRD) after consolidation, with a planned allogeneic transplant for MRD-positive patients (≥0.001%). Treatment abandonment was defined as a missed ≥14-day period during intensive treatment or ≥1 month during maintenance. Statistical analysis was performed as intent-to-treat. Lastly, drugs included in our protocol were compared to those of CALGB 10403 with current local pricing in USD.
Results
Ninety-one AYAs have been treated, 47 women and 44 men, with a median age of 21 years (range, 15-45), mostly with a good functional capacity and no comorbidities (ECOG≤2: 92.1%; HCT-Ci 0-1: 97.8%); 43.8% of patients had ≥30x10 9/L white blood cells at diagnosis and 31.7% had grade ≥1 obesity. Notable grade ≥3 adverse events during induction were infections/neutropenic fever (35.6%),hepatotoxicity (11%) and thrombosis/bleeding (8.1%) with 44.3% eventually requiring hospitalization. Induction related mortality was 11%. Only n=3 were refractory to induction and the remainder assessed achieved complete remission (n=63; 95.5%) with a median follow-up of 15 months (range, 0.9-50.1). N=29 received induction and consolidation entirely on an outpatient basis, ulterior hospitalizations during therapy were rare. Treatment abandonment was common (n=24; 26.4%) usually during induction (n=8; 32%) or consolidation (n=12; 48%) and mostly related to unaffordability. For the same reason, transfers to social security healthcare systems were also frequent (n=19; 20.9%). Most patients assessed were MRD negative (n=38; 74.5%) Early relapse incidence was 32.9%; 44.4% in MRD-positive and 27.5% in MRD-negative patients (p=0.43). OS at 24 months was 61.5% (95% CI 47-73%) and EFS 49.8% (95% CI 37-62%) with excellent outcomes for MRD-negative patients (Figure 1, Panels A and B). Treatment abandonment and MRD positivity were the only independent predictors of mortality in a multivariate analysis (HR 7.8 [95% CI 2.8-21.9] and HR 4.5 [95% CI 1.4-14], respectively). Lastly, the total cumulative price for medications included in our regimen was calculated at $16,750 vs. $36,061 USD in CALGB 10403, representing a cost reduction of 53.5%.
Conclusions
The treatment of Hispanic ALL patients with our regimen has shown promising outcomes at a reduced cost for patients. Genetic risk assessment, induction mortality, treatment abandonment and lack of access to novel therapies for MRD positive patients remain the main barriers for improving outcomes further.
Gomez-De Leon: Novartis: Honoraria; ASH: Research Funding; Sanofi: Honoraria; Abbvie: Honoraria. González López: JANSSEN: Honoraria; AMGEN: Honoraria. Gomez-Almaguer: Roche: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Bristol-Myers-Squibb: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal